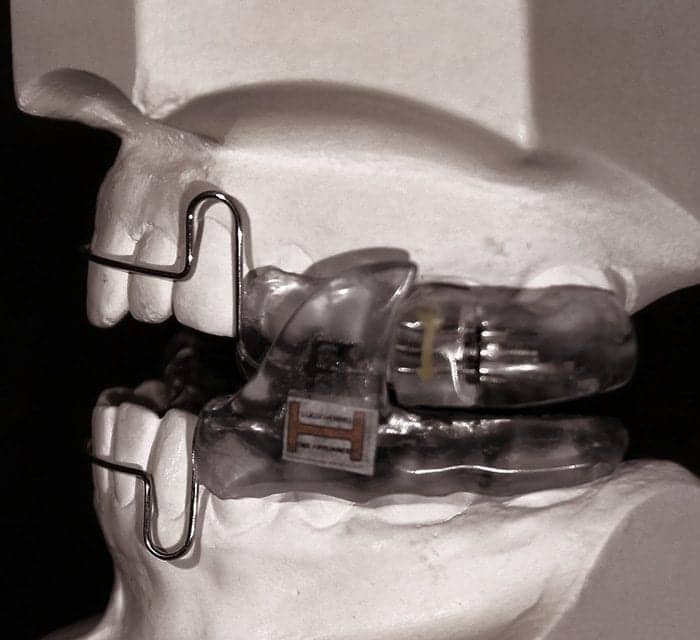
The Food and Drug (FDA) has just market cleared the Luco Hybrid OSA Appliance for its 2nd 501K. K160477 was granted July 29 for the treatment of sleep bruxism and to aid in the treatment of associated tension/migraine type headaches in adults.
The Luco appliance indications for use to date are:
- For the treatment of primary snoring , mild to moderate obstructive sleep apnea in adults (K130797)
- For the treatment of sleep bruxism in adults (K160477)
- As an aid in the treatment of associated tension/migraine type headaches in adults (K160477)