The benefits of lighting that helps us sleep, improves our mood, reduces depression, or makes us feel more alert on the job are, simply put, priceless. An expert from the Lighting Research Center at Rensselaer Polytechnic Institute explains how to make light therapy successful in clinical settings.
Light is not just for vision. Humans have a biological clock located in the suprachiasmatic nuclei that generates and regulates circadian rhythms, which are biological rhythms that repeat approximately every 24 hours. These include cycles such as sleep-wake, body temperature, hormone production, and alertness. Light is the main input to synchronize the biological clock to the solar day. If we are not exposed to a sufficient amount of light of the right spectrum, for a sufficient amount of time, and with the right timing, our biological clock becomes desynchronized with the solar day and we may experience decrements in physiological functions, neurobehavioral performance, and sleep.1,2
A person is more likely to experience a good night of sleep when the circadian and homeostatic systems, both of which influence the sleep-wake cycle, are aligned. Sleep homeostasis increases with time awake, contributing to high sleep pressure at night. The circadian system sends an alerting signal to the body during the day, counteracting the increase of sleep pressure with time awake, and a sleeping signal during the night, promoting a consolidated night of sleep.
Another very well-known circadian rhythm is the cycle of melatonin production. Melatonin is a hormone produced by the pineal gland at night and under conditions of darkness. For diurnal species, such as humans, melatonin signals that it is time to sleep. The timing of melatonin onset in the evening, referred to as dim light melatonin onset (DLMO), occurs approximately 2 hours prior to natural bedtimes, and is used as a marker of the circadian clock. Evening exposure to sufficient light will delay the onset of melatonin, delaying bedtimes.
Lighting Characteristics Affecting the Circadian System
Lighting characteristics affecting the circadian system, as measured by acute melatonin suppression and phase shifting of DLMO, are different than those affecting visibility. It is now known that lower levels of light, less than those originally demonstrated in the 1980s, can affect melatonin; however, light levels needed to affect melatonin are still higher than those needed to affect vision.3,4 For example, a warm color night light will allow one to safely navigate at night, but it will not suppress the hormone melatonin. Humans are “blue sky detectors” when it comes to acute melatonin suppression; the peak sensitivity for acute melatonin suppression and phase shifting of DLMO is close to 460 nanometers (nm).5-7
The effects of light on the circadian system vary over the course of the 24-hour day. Morning light, given after the trough of core body temperature that typically occurs in the second half of the night, will advance the timing of sleep in the following cycle, while evening light, given prior to the trough of core body temperature, will delay the timing of sleep.8
Finally, our research shows that it is important to accurately measure light exposures over the 24-hour day, as opposed to taking just a “snapshot” measurement of light exposure at one certain place and time.9,10 The circadian system seems to keep track of light exposure, so that knowing an individual’s light exposure history over the past 24 hours can help determine the best light prescription for the next 24 hours.11 Therefore, a light treatment designed to promote earlier bedtimes should not be limited to exposure to blue light in the morning. It should control the total circadian light exposure during waking hours.
Potential Applications
Keeping in mind that lighting characteristics affecting the circadian system are different than those affecting vision, and, more importantly, that the successful application of light as therapy requires continuous monitoring of one’s light exposure during waking hours, the therapeutic value of “circadian” light (and dark) on special populations has been demonstrated in laboratory and field studies.
Seasonal Affective Disorder
Seasonal affective disorder (SAD) is a subtype of depression, with episodes occurring during winter months and remitting during summer months. The mechanisms of SAD are still unknown, and there are several competing hypotheses as to what causes SAD and how light can be used as a treatment.
One of these is that late daybreak during winter months delays the circadian rhythms of those more susceptible to SAD; in this case, morning light is believed to be effective in treating symptoms of SAD.
Another hypothesis is that the overall melatonin production of those suffering from SAD is greater during winter months than during summer months, which extends the amount of time during the 24-hour day that their bodies think it is nighttime. In this case, light in the early morning or evening is recommended.
While current practice is to use 2,500 lux at the cornea of a white “full spectrum” light box for 2 hours or 10,000 lux at the cornea for 30-minute exposure, more recent studies demonstrated the effectiveness of lower levels (<500 lux at the cornea) of 470 nm (blue light) at improving SAD symptoms.12,13 The use of a more tailored spectrum (ie, increased emission in the short-wavelength region) allows for a reduction in the light level required to elicit the same benefit, which makes the therapy more comfortable, likely increasing compliance.14
Sleep in Older Adults
Seniors residing in assisted living facilities are perhaps the best example of a population at risk for circadian disorders. Due to age-dependent reduced retinal light exposures and to fixed lighting conditions in their living environments, seniors are less likely to experience the necessary robust 24-hour light-dark pattern needed for circadian entrainment. Exposure to bright white light (at least 3,000 lux and as high as 8,000 lux at the cornea) for at least 1 hour in the morning for a period of at least 2 weeks was found to improve or consolidate nighttime sleep of individuals with Alzheimer’s disease and related dementias (ADRD).15,16 Research demonstrated that, in ADRD patients, continuous, bright, indirect white light (at least 1,000 lux at the cornea) during daytime hours consolidated rest-activity rhythms,17 attenuated cognitive deterioration, ameliorated depressive symptoms, and attenuated the increase in functional limitations over time.18 Recently, we showed that much lower levels (400 lux at the cornea) of a high correlated color temperature light source (which emits more short-wavelength content) improved objective and subjective sleep and reduced agitation and depression in ADRD patients living in long-term care facilities.14

The newly constructed 24-hour lighting scheme demonstration room at the Lighting Research Center (LRC) at Rensselaer Polytechnic Institute provides cycled electric lighting with cool, high light levels during the day and warm, low levels in the evening. Construction of the room was made possible through support from the Light & Health Alliance at LRC. Light & Health Alliance members include Acuity Brands, Ketra, GE Lighting, OSRAM Sylvania, Philips Lighting, Sharp, and USAI Lighting.
A 24-hour lighting scheme has been proposed19 that delivers high circadian stimulation during the daytime hours, low circadian stimulation in the evening hours, and night lights that provide perceptual cues to decrease the risk of falls at night without disrupting sleep. Arguably, light therapy in assisted living facilities should be quite effective because it is possible to fully control the 24-hour light-dark pattern, as residents spend the majority of their time indoors in a defined space.

Night lights providing horizontal and vertical visual cues reduce fall risk without disrupting sleep.
Sleep in Shift Workers
Light can impact circadian rhythms of night shift workers in two ways: phase shifting and acute effects. Phase shifting effects allow permanent night shift workers to cope with being awake at night by providing entrainment to the night shift. However, light exposure control throughout the 24-hour day is needed, making it harder for workers to comply with the new light regimen.
Light can increase alertness in shift workers who are still entrained to a day shift schedule and are coping with night shift work. In most studies to date, the alerting effects of light have been linked to its ability to suppress melatonin, and the suppression of melatonin by light at night has been implicated as an endocrine disruptor and linked to an increased risk of certain types of cancers observed in those working rotating shifts for 20 to 30 years or more.20,21 In a series of recent studies, however, we demonstrated that exposures to both short-wavelength (blue) and long-wavelength (red) lights in the middle of the night increased objective and subjective alertness, reduced subjective sleepiness, and improved certain types of performance tasks, mainly those associated with reaction times.22-24 Our findings suggest that the melatonin pathway does not seem to be the only light-sensitive pathway that can affect alertness at night. These results are consistent with studies showing alerting effects of light during the daytime, when melatonin levels are low.25,26
Although more research is recommended to establish the pathways in the brain associated with the alerting effects of long-wavelength light, using red, rather than blue, light at night might be a good option for increasing alertness without disturbing the melatonin rhythm in shift workers.
Sleep in Adolescents
Teenagers tend to go to sleep late and wake up late. This pattern interferes with their normal functioning because teenagers do not sleep for as many hours as those going to sleep at more normal hours. Light exposure after minimum core body temperature (in the morning) and light restriction in the evening have been shown to advance the phase of the master clock.27 Some evidence has emerged suggesting that the endogenous circadian period is longer, sleep pressure accumulates slower, and the sensitivity to light by the circadian system is greater in adolescents than in adults, especially for those suffering from delayed sleep phase disorder. In fact, recent data from our lab show that adolescents (ages 15-17 years) are more sensitive to evening light from self-luminous devices than those in their twenties.28 Both of these phenomena would functionally delay the timing of the adolescent circadian clock, thereby contributing to later bed and rise times.
Rigid school schedules require teens to be in class all morning, yet schools may not provide adequate light or daylight to stimulate their circadian system, especially in dark winter months. As teenagers spend more time indoors, they may miss out on essential morning light needed to stimulate their circadian system and promote entrainment to the 24-hour solar day.29 In late spring, if teenagers spend more time outdoors after school, the brain’s clock may be delayed by the evening daylight exposure.30 Late spring mornings may also pose a concern because of the very early sunrises. Students who are phase delayed, and thus reach their minimum core body temperature at later clock hours in the morning, may run the risk of receiving too much daylight in the delay portion of the phase response curve while traveling to school in the very early morning.
A lighting intervention designed to deliver light starting mid-morning (eg, after 9 AM) until the end of the school day and provisions to reduce evening light exposures, such as the use of orange-tinted glasses and the control of exposure to self-luminous devices, may help shift the timing of sleep in teenagers. Successful light therapy in this population will likely involve both school administrators and family members.
How Do We Make Light Therapy Successful in Clinical Settings?
The key to successful light therapy is simple: We need to measure our circadian light exposures and then deliver and remove circadian light at the appropriate circadian times.
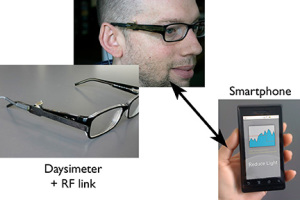
The Daysimeter, developed by the LRC as a research tool, is a calibrated, personal light and activity sensor that continuously records and stores data for measuring circadian light and for quantifying circadian entrainment or disruption. In the prototype system shown here, the Daysimeter communicates with a smartphone, which, in turn, communicates with building lighting control systems to deliver prescribed light treatments to entrain or realign circadian rhythms to support health and well-being.
However, this is easier said than done. We have no practical way to intuitively know when and what type of light is needed for circadian entrainment. Like other medical monitors where we have no conscious access to what is required for good health (eg, glucose monitors for diabetes), we need technologies to measure and track the state of our circadian system. A wireless technology connecting the Daysimeter10 to a smartphone is currently under development. A smartphone (or similar technology) will be able to interpret the light-dark patterns to which an individual is exposed and then recommend, based on models of human circadian entrainment,7,31 current and future light exposure patterns to maintain circadian entrainment. One day, the smartphone or a smartwatch might be able to interface with building lighting systems so that children at schools, nurses at hospitals, or people at home would receive the proper lighting conditions for improved sleep and overall well-being. Until then, if we want to have a good night of sleep, we should keep a regular schedule, increase the amount of circadian light exposure during the daytime hours, and reduce it during the evening hours.
Mariana G. Figueiro, PhD, is light and health program director at the Lighting Research Center and professor at Rensselaer Polytechnic Institute. She is the author of more than 60 scientific articles in her field of research, along with the AARP-sponsored publication, Lighting the Way: a Key to Independence, which provides guidelines for the design of lighting to meet the needs of older adults.
References
1. Leproult R, et al. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860-9.
2. Van Cauter E, et al. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9:S23-8.
3. Lewy A, et al. Light suppresses melatonin secretion in humans. Science. 1980;210:1267-9.
4. Zeitzer JM, et al. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695-702.
5. Brainard GC, et al. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405-12.
6. Thapan K, et al. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261-7.
7. Rea MS, et al. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50:213-28.
8. Khalsa SB, et al. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945-52.
9. Rea MS, et al. A new approach to understanding the impact of circadian disruption on human health. J Circadian Rhythms. 2008;6:7.
10. Figueiro MG, et al. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Light Res Technol. 2013;45:421-34.
11. Figueiro MG, et al. Daylight exposure has a positive carry-over effect on nighttime performance and subjective sleepiness. Light Res Technol. 2014;46:506-19.
12. Glickman G, et al. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes. Biol Psychiatry. 2006;59:502-7.
13. Strong RE, et al. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26:273-8.
14. Figueiro MG, et al. Tailored lighting intervention improves measures of sleep, depression and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527-37.
15. Mishima K, et al. Randomized, dim light controlled, crossover test of morning bright light therapy for rest-activity rhythm disorders in patients with vascular dementia and dementia of Alzheimer’s type. Chronobiol Int. 1998;15:647-54.
16. Yamadera H, et al. Effects of bright light on cognitive and sleep–wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54:352-3.
17. Van Someren EJW, et al. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955-63.
18. Riemersma-van der Lek RF, et al. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299:2642-55.
19. Figueiro MG. A proposed 24 h lighting scheme for older adults. Light Res Technol. 2008;40:153-60.
20. Schernhammer ES, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93:1563-8.
21. Schernhammer ES, et al. Night-shift work and risk of colorectal cancer in the Nurses’ Health Study. J Natl Cancer Inst. 2003;95:825-88.
22. Figueiro MG, et al. Preliminary evidence that both blue and red light can induce alertness at night. BMC Neurosci. 2009;10:105.
23. Figueiro MG, et al. Light at night and measures of alertness and performance: Implications for shift workers. Biol Res Nurs. 2014; doi: 10.1177/1099800415572873.
24. Plitnick B, et al. The effects of red and blue light on alertness and mood at night. Light Res Technol. 2010;42:449-58.
25. Sahin L, Figueiro MG. Alerting effects of short-wavelength (blue) and long-wavelength (red) lights in the afternoon. Physiol Behav. 2013;116-117:1-7.
26. Sahin L, et al. Daytime light exposure: Effects on biomarkers, measures of alertness, and performance. Behav Brain Res. 2014;274:176-85.
27. Figueiro MG, et al. The effects of chronotype, sleep schedule and light/dark pattern exposures on circadian phase. Sleep Med. 2014;15:1554-64.
28. Figueiro MG, Overington D. Self-luminous devices and melatonin suppression in adolescents. Light Res Technol. 2015. In Press.
29. Figueiro MG, Rea MS. Lack of short-wavelength light during the school day delays dim light melatonin onset (DLMO) in middle school students. Neuroendocrinol Lett. 2010;31:4.
30. Figueiro MG, Rea MS. Evening daylight may cause adolescents to sleep less in spring than in winter. Chronobiol Int. 2010;27:1242-58.
31. Rea MS, et al. Field tests of a model of the human circadian oscillator. SLEEP 2014. Minneapolis; 2014.






