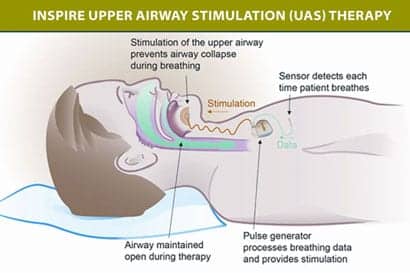
In a study of 126 moderate-to-severe obstructive sleep apnea (OSA) patients with a history of noncompliance with CPAP, Inspire’s implanted upper-airway stimulation device led to significant improvements in objective and subjective measurements of OSA severity. Published in the New England Journal of Medicine, the year-long study implanted the patients with the device. The stimulator is implanted via surgery, during which the stimulation electrode is placed on the hypoglossal nerve to recruit tongue-protrusion function; the sensing lead is placed between the internal and external intercostal muscles to detect ventilatory effort; and the neurostimulator was implanted in the right ipsilateral mid-infraclavicular region.
In the study, the median AHI score at 12 months decreased 68%, from 29.3 events per hour to 9 events per hour, and the ODI score decreased 70%, from 25.4 events per hour to 7.4 events per hour. Secondary outcome measures showed a reduction in the effects of sleep apnea and improved quality of life.
Two participants had a serious device-related adverse event requiring repositioning and fixation of the neurostimulator to resolve discomfort. A total of 33 serious adverse events not considered to be related to the implantation procedure or implanted devices were reported. Most nonserious adverse events related to the procedure (88%) occurred within 30 days after implantation and were expected postsurgical events, including sore throat from intubation, pain at the incision site, and muscle soreness.
Inspire Medical Systems is in the process of seeking approval from the FDA for use of Inspire therapy for patients with moderate to severe OSA who are intolerant to CPAP.