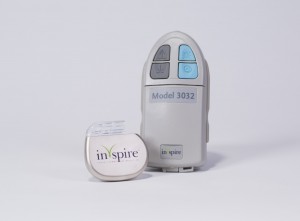
The Food and Drug Administration (FDA) has approved Inspire Medical‘s Upper Airway Stimulation (UAS) therapy for use in a subset of patients with moderate to severe obstructive sleep apnea (OSA) who are unable to use CPAP. Inspire therapy is a fully implanted neurostimulation device, the first of its kind for sleep apnea, that has been shown to significantly approve OSA.
Inspire therapy is a fully implanted system consisting of three components: a small generator, a sensing lead, and a stimulation lead. The single external component is a small handheld Inspire sleep remote used to turn the therapy on before bed and off upon waking. When activated, Inspire therapy senses breathing patterns and delivers mild stimulation to key airway muscles, which keeps the airway open during sleep. In contrast to other surgical options to treat sleep apnea, Inspire therapy does not require removal or permanent alteration of facial or airway anatomy. As such, the procedure is less invasive and should result in a shorter recovery time.
“The FDA approval of Inspire therapy represents a new era of choice for a subset of patients with moderate to severe Obstructive Sleep Apnea who are unable to use CPAP,” says Tim Herbert, Inspire Medical Systems president and CEO, in a release. “All of us at Inspire Medical Systems are committed to improving the health and quality of life for these individuals with OSA, and we are excited to make this innovative and much needed treatment available to patients and physicians.”
“This therapy represents a major advance in sleep apnea treatment for some patients who are unable to use or tolerate CPAP therapy,” says Meir Kryger, MD, professor, Yale School of Medicine. “Patients with moderate to severe OSA who are not on effective treatment are at an increased risk for cardiovascular disease, accidents, and death. There is a significant need for safe, effective, and well-tolerated new treatments in the sleep medicine field.”
Patients implanted with Inspire therapy who participated in the company’s STAR (Stimulation Therapy for Apnea Reduction) pivotal clinical trial experienced a 68% reduction in apnea events, a 70% reduction in oxygen desaturation events, and significant improvements in daytime functioning as measured by two validated questionnaires. These results were published in the January 9, 2014 issue of the New England Journal of Medicine.
Inspire therapy will be commercially available to patients in the United States in the second half of 2014.