It’s easier than ever to go beyond a sleeping pill prescription.
By Lisa Spear
When sleep specialist Michelle Primeau, MD, takes out her prescription pad for a patient with insomnia, she is likely not prescribing a sleeping pill like Ambien but directing patients to a software program instead.
Though many people with insomnia go into doctor’s visits expecting a drug to relieve their sleeplessness, stakeholders are moving increasingly toward holistic approaches. Clinicians, medical technology firms, and even pharmaceutical companies are seeing the benefits of treating the whole person. Primeau has spent years recommending SHUTi, recently relaunched as Somryst, the first prescription digital therapeutic intended for use in the treatment of patients 22 years of age and older with chronic insomnia.
“We are developing software as treatment,” says Yuri Maricich, MD, MBA, chief medical officer and head of development at Pear Therapeutics, which recently received US Food & Drug Administration (FDA) approval for Somryst. “This is a whole treatment modality that is starting to be integrated into standard care.”
Now is a particularly opportune time for a more holistic approach to insomnia treatment. In 2019, the FDA began requiring that some common prescription sleep aids (those containing eszopiclone, zaleplon, or zolpidem) carry a black-box warning about the risk of rare but serious injuries and deaths resulting from various complex sleep behaviors after taking these medicines.1 The American Academy of Sleep Medicine in 2020 issued a clinical practice guideline on behavioral and psychological treatments for insomnia in adults. It includes several recommendations suggesting clinicians use multi-component brief therapies and three single-component therapies: stimulus control, sleep restriction therapy, and relaxation therapy.2 While there is a shortage of behavioral sleep specialists in the United States, computer-based programs for cognitive behavioral therapy for insomnia (CBT-I) have cropped up as the technology to deliver them improves.
“One of the biggest things is that it helps to improve access for patients,” says Primeau, medical director of the Palo Alto Medical Foundation Sleep Medicine Center in San Carlos, Calif.
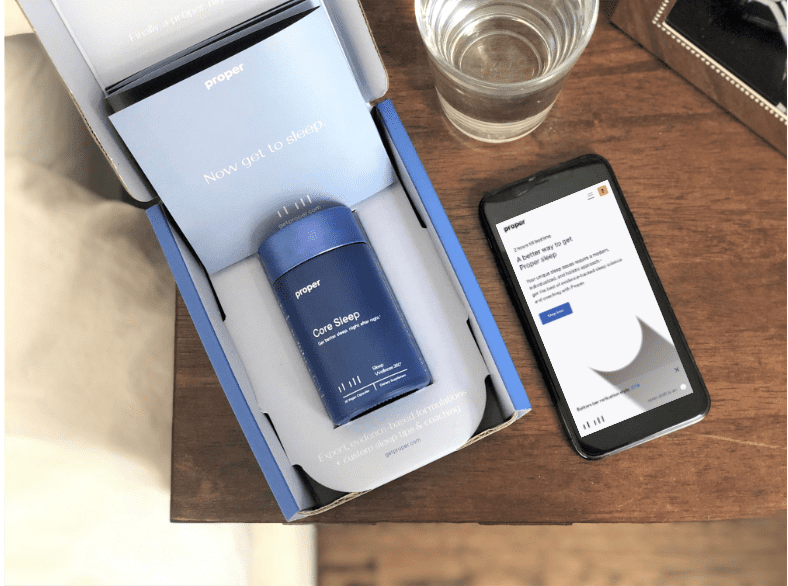
Another service to address sleeplessness holistically is the startup Proper, which launched in 2020 as a personalized subscription program with behavioral sleep coaching and supplement recommendations. Proper’s slogan is “sleep isn’t one-size-fits all” and the company sells a mix of over-the-counter supplements (with ingredients including valerian root extract, extended-release melatonin, vitamins, and cannabidiols).
“While we believe in formulations, we’d never mistake them for a one-size-fits-all ‘magic pill’ that will solve everyone’s sleep issues. That’s where our 1:1 digital coaching comes in—with considerations for our customers’ overall lifestyle and health….Our professional sleep coaches deliver individualized tools and advice in the form of what we call a ‘sleep action plan’ (a personalized set of recommendations, techniques, and guidelines…tailored to customers individual needs) to prime them for a Proper night’s sleep night after night,” says CEO Nancy Ramamurthi.
Proper’s sleep coaching process starts with an online assessment, an intake questionnaire that allows sleep coaches to better prepare for the consultation to tailor it to the person’s specific needs and goals.
During the session, which is conducted via Zoom or phone, Proper’s coaches focus on creating more awareness around behavioral and environmental sleep habits. “The goal of these sessions is to develop a comprehensive, personalized sleep action plan,” says Ramamurthi. “For example, for someone dealing with everyday stress, it may include a breathing exercise for them to practice when falling asleep to clear a racing mind or may recommend journaling before bed to get rid of the ongoing mental to-do list that often keeps people from falling asleep,” says Ramamurthi.
Pharmaceutical companies too have launched behavioral tools to supplement their prescription insomnia medications. Merck, marketer of Belsomra, has a “Belsomra Beginnings” website that includes a guide to meaningful conversations with a doctor as well as a sleep habits quiz. Japanese company Eisai, marketer of the insomnia medication Dayvigo, has made several interactive tools available, including a website that supplies patients with ideas about how to create a relaxing bedroom environment and establish a bedtime routine.
“If you think about sleep disorders, and insomnia particularly, there really is a need for a holistic approach,” says Tushar Patel, PhD, vice president of US sleep-wake commercial and global strategy at Eisai. “We fully recognize than nobody wants to be on sleep medication day in and day out every night to go to bed. So if we can really help patients understand what is causing the difficulty with sleeping, many may still need to be treated with medication, but many may be able to have better sleep without the need for drug therapy.”
[RELATED: Massage Therapy for a Better Night’s Sleep]
As part of the company’s effort to help patients better understand their sleep, Eisai also created an Amazon Alexa sleep tracker called “My Sleep Log.” Instead of writing in a traditional paper sleep diary, the patient asks the AI-driven virtual assistant to “open my sleep log.” The program is designed to help people more easily keep tabs on their sleep schedule.
Patel says these new holistic programs have become part of the company’s DNA.
“Good business for us is that we are doing right by the patient,” he says. “If you are helping the patient try to understand their condition, how to manage their condition, how to identify triggers, that is good business.”
Lisa Spear is associate editor of Sleep Review.
Somryst Leads the Way for Digital Health Software FDA Pathway
Boston-based biotech company Pear Therapeutics develops health software in the same way a pharmaceutical company creates a new drug. Its software programs are tested for efficacy through randomized clinical trials, then the data from the trials is submitted to the FDA for approval.
Pear Therapeutics is one of nine companies taking part in the FDA’s Digital Health Software Precertification Pilot Program. Its insomnia software, Somryst, was submitted, reviewed, and cleared through the traditional 510(k) pathway and was the first product reviewed through FDA’s Software Precertification Pilot Program, as part of the 2019 Test Plan released by the FDA in January 2019. In March 2020, the FDA granted authorization for Somryst to be marketed as a digital therapeutic intended for use in the treatment of patients 22 years of age and older with chronic insomnia.
“While we just launched Somryst commercially, there have been thousands of patients who have used it in research studies,” says Yuri Maricich, MD, MBA, chief medical officer and head of development at Pear Therapeutics.
Those research studies show lasting improvements in sleep at 12 and 18 months for people experiencing chronic insomnia. In clinical studies of more than 1,400 adults with chronic insomnia, Somryst reduced the amount of time it took to fall asleep by 45%, reduced the amount of time spent awake at night by 52%, and reduced the severity of insomnia symptoms by 45%, with continued improvement at 6 and 12 months post-treatment. Results from clinical studies have been published in JAMA Psychiatry and Lancet Psychiatry.3, 4, 5
Somryst is currently only available to select providers and medical centers, but the software will be rolled out in 2021 to all licensed healthcare providers. Like pharmaceutical sleep aids, clinicians must first evaluate the patient before they write a prescription for the software. Then patients can access the platform from home by downloading it on Google Play or the Apple App Store.
Over about 9 weeks, depending on a patient’s pace, Somryst takes them through short lessons and challenges, including algorithm-driven sleep restriction, to train the brain and body to improve their sleep. Progress is measured by using the Insomnia Severity Index. The software also tracks self-reports of the time it takes for the patient to fall asleep as well as their nocturnal awakenings.
“The patient does this fully on their mobile device, so it is not telemedicine. All the treatment is fully automated. It’s a combination of video images, interactive exercises, text, and there’s also an algorithm that helps make recommendations on the patient’s sleep window as they input their sleep metrics over the course of their treatment,” says Maricich. —Lisa Spear
References
1. FDA Drug Safety Communication. FDA adds Boxed Warning for risk of serious injuries caused by sleepwalking with certain prescription insomnia medicines. 30 Apr 2019. Available at https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-risk-serious-injuries-caused-sleepwalking-certain-prescription-insomnia.
2. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2020 Nov 9. Online ahead of print.
3. Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinicals trial. JAMA Psychiatry. 2017;74(1):68–75.
4. Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3:333–41.
5. Batterham P, Christensen H, Mackinnon A, et al. Trajectories of change and long-term outcomes in a randomized controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017;3(5):228-35.




