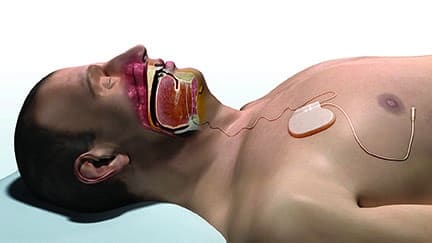
Apnex Medical Inc has received CE Mark approval for its Hypoglossal Nerve Stimulation (HGNS) System for use by people who suffer from obstructive sleep apnea (OSA).
The system was approved for sale in Europe based on the positive results of two clinical studies conducted in the United States and Australia. In those studies, the majority of patients demonstrated a significant reduction in their obstructive sleep apnea as well as substantial improvements in the quality of their sleep, quality of life, and overall health.
"CE Mark approval is an important confirmation of the substantial benefits that patients receive from our HGNS therapy for obstructive sleep apnea and is a key milestone for our company," said Chas McKhann, Apnex Medical President and CEO. "We are excited to bring this innovative new therapy to Europe. We are also focusing on further evaluation of the HGNS System in the Apnex Clinical Study, a randomized clinical trial of the HGNS system that is enrolling patients in the United States, Australia, and select countries in Europe."
Apnex Medical Inc received investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to conduct a clinical study to evaluate the safety and effectiveness of its HGNS System in people who suffer fromOSA. Data from this clinical study is intended to support the Pre-Market Approval (PMA) application for the HGNS System to the FDA.