Patients can benefit from polysomnography when there is evidence of chronic obstructive pulmonary disease and obstructive sleep apnea
It can be argued that the term COPD should be used only for patients who have airway obstruction manifested either clinically or as an abnormality in a standard spirometry index, such as forced expiratory volume in 1 second (FEV1). COPD may also be defined as a process characterized by the presence of chronic bronchitis or emphysema that may lead to the development of airway obstruction that may be partially reversible. The airway obstruction is generally progressive. Appropriate evaluation of COPD patients generally includes clinical assessment, radiography, pulmonary function tests, and laboratory tests.
Tobacco smoking accounts for 80% to 90% of the risk of developing COPD in the United States.4 The risk is strongly associated with the intensity and the duration of smoking. Other factors that increase the risk of COPD include occupational or dust exposure, environmental air pollution, a1-antitrypsin deficiency, a history of childhood respiratory infection, advanced age, and factors related to low socioeconomic status.
Symptoms of COPD
Patients usually present with some combination of cough, dyspnea, and wheezing. The symptoms are less common and less severe in the early stages of disease. Severity of symptoms is poorly correlated with the severity of airflow limitation. Flow limitation should be documented by spirometry data. A reduced ratio of FEV1 to forced vital capacity (FVC) confirms the presence of airflow obstruction. The level of FEV1 is the best predictor of severity and survival. Airflow obstruction in COPD is generally progressive and is largely (mostly) irreversible. The decline of FEV1 in people who have never smoked is 19 to 52 mL per year; in smokers, it is 34 to 79 mL per year.5 In patients who continue to smoke, the decline of FEV1 is 62 mL per year, and in those who successfully stop smoking, it is 31 mL per year.6 The patient’s single-breath diffusing capacity is decreased in proportion to the severity of emphysema due to loss of the capillary bed. As the disease progresses, hypoxemia occurs, and hypercapnia is seen in advanced disease (FEV1 of less than 1 L). The relationship between arterial–blood-gas (ABG) levels and empiric spirometry values is weak. ABG abnormalities usually worsen during exercise and sleep.
COPD and its Effect on Sleep
In several studies,7,8 the prevalence of nocturnal hypoxemia in COPD is reported at 24% to 27%. Desaturation is more pronounced in patients with low ventilation-perfusion ratios and decreased ventilatory drive (blue bloaters) than in those with higher ventilation-perfusion ratios and normal ventilatory drive (pink puffers). Lung function is not a predictor of sleep desaturation, which is more frequent in patients who are smokers, who complain of sleepiness, and who have lower hypercapnic ventilatory responses. Mechanisms of sleep-induced hypoxemia may be related to hypoventilation due to worsening mechanics, worsening ventilation-perfusion mismatching, decreased hypoxic respiratory drive, decreased hypercapnic respiratory drive, respiratory dysrhythmia of rapid–eye-movement (REM) sleep, decreased respiratory muscle activity (especially in REM sleep), increased upper-airway resistance, obstructive sleep apnea (OSA), and decreased functional residual capacity (FRC).
The term “overlap syndrome” applies to patients with coexisting COPD and OSA. The prevalence of COPD in patients with OSA was reported as 11%.9 Prevalence of OSA in a preselected group of COPD patients who had symptoms of OSA was reported as 20%.10 In some small series,11 the prevalence of OSA in COPD patients was suggested as being as high as 40%. Factors that explain the high prevalence of overlap syndrome are similar patient demographics, with older males being particularly affected by both disorders; smoking, which is also a risk factor for OSA; and a defect in control of breathing that is seen in some COPD patients and that may also be responsible for OSA.12
The sequelae of recurrent hypoxemia in patients with COPD may be pulmonary hypertension, cor pulmonale, polycythemia, cardiac-rhythm disturbances, and sleep complaints. OSA may hasten the onset of ventilatory failure in COPD. In one study, 42% of patients with overlap syndrome had pulmonary hypertension.9
Sleep-related symptoms include sleep-onset insomnia and sleep-maintenance insomnia, as well as daytime somnolence. Whether these are related to COPD symptoms such as nocturnal cough and wheezing, to medications such as theophylline and salmeterol, and/or to arousals related to frequent desaturation and respiratory events in sleep is unknown.
Pharmacological agents such as salmeterol and protriptyline do not improve nocturnal oxygenation. In several studies,13,14 theophylline use resulted in improved nocturnal oxygenation, but its side effects of cardiac dysrhythmia and impaired quality of sleep mitigated the small benefit of improved oxygenation.
Oxygen Therapy
COPD patients with hypoxemia have a poor prognosis despite aggressive medical treatment to improve the mechanics of the lungs and the airway inflammation.
Long-term oxygen therapy has become one of the major forms of treatment for hypoxemic COPD patients; it is generally held to result in decreased pulmonary hypertension, improved exercise tolerance, decreased erythrocytosis, and improved neuropsychological function.
The Medical Research Council trial15 in the United Kingdom evaluated hypoxemic COPD patients (with Pao2 levels of 40 to 60 mm Hg, with a mean of 50.4 mm Hg) in a randomized study in which they received continuous oxygen therapy or no supplemental oxygen. The study showed a survival advantage of 5 years when continuous oxygen was administered. These patients had FEV1 results of 0.76 to 0.58 L. Patients also reported general improvement in well-being and appetite.16
In the United States, a randomized study8 of hypoxemic COPD patients evaluated the use of continuous oxygen versus nocturnal oxygen therapy; 203 patients were randomly assigned to receive either continuous oxygen (17.7 hours per day or more) or 12 hours of nocturnal oxygen. The subjects were followed for at least 1 year (mean: 19.3 months). Entry criteria included Pao2 of less than 55 mm Hg or Pao2 of less than 59 mm Hg with edema; hematocrit of more than 55%; or a P pulmonale wave visible on an electrocardiogram. Oxygen was provided at the lowest flow rate that improved Pao2 to 60 to 80 mm Hg. Flow was increased by 1 L/min for exercise and sleep.
Compliance was reported as very good in both groups. Overall, mortality in the continuous therapy group was less (11.9%) than in the nocturnal oxygen group (20%). When mortality was examined in a subgroup of patients whose arterial Paco2 was more than 43 mm Hg, survival was better in the continuous–oxygen-therapy group (P<.002). In patients with low pH and high levels of mood disturbance such as depression and anxiety, the continuous–oxygen-therapy group demonstrated significantly greater survival than the group receiving nocturnal oxygen therapy. The patients with severe nocturnal hypoxemia, low hematocrit, and/or severe brain dysfunction had lower mortality with continuous oxygen therapy than with nocturnal oxygen therapy. Some decrease in hematocrit was seen within 6 months, but a significant reduction was seen after 12 months in polycythemic patients. Hospitalizations were reported less often for the group receiving continuous oxygen therapy.
Overall, patients with hypoventilation, poor lung function, high Paco2, low pH, significant nocturnal oxygen desaturation, low FVC, high FRC, or more severe neuropsychological dysfunction showed the largest survival advantage for continuous oxygen therapy.
Studies
In severely hypoxemic COPD patients, a reduction in pulmonary vascular resistance or mean pulmonary artery pressure, an increased stroke-volume index, and a decreased mean right atrial pressure were noted after 6 months in the continuous–oxygen-therapy group.17 It is unclear whether these differences explained the differences in mortality between these two groups.
In another British trial18 of 15-hour oxygen therapy per day (compared with no oxygen therapy) in severe hypoxemic COPD, patients showed a lower mortality in the treated group. One can conclude that some oxygen is better than none, and that continuous oxygen therapy (more than 19 hours per day) is superior to nocturnal oxygen therapy only in severely hypoxemic COPD patients.
Gorecka et al19 did not observe any survival advantages in 67 patients with COPD and moderate hypoxemia (Pao2 of more than 65 mm Hg) who were randomized to 17 hours per day of oxygen therapy versus a control group (after a follow-up period of 85 months). In several studies9,20 of nocturnal oxygen therapy in patients with mild-to-moderate hypoxemia, no survival benefit was documented.
Fletcher et al20 studied 38 COPD patients with Pao2 above 60 mm Hg and sleep-related oxygen desaturation. In a controlled, randomized, double-blind study, no differences in mortality were seen after 36 months between the oxygen-therapy group and the control group; however, they reported some hemodynamic improvement in the oxygen-therapy group.
Chaoust et al9 evaluated 30 patients with COPD and mild-to-moderate hypoxemia (Pao2 of 56 to 69 mm Hg) and significant nocturnal oxygen desaturation. Patients with comorbidity (congestive heart failure or OSA) were excluded. In randomized studies, patients were treated with oxygen for 8 to 10 hours per night (versus no treatment). No survival benefit was noted in the treated group. Nocturnal oxygen did not delay the need for long-term oxygen therapy (24 hours per day). No improvement in hemodynamics was reported in this study.
In summary, a significant survival advantage is noted in selected COPD patients who receive continuous long-term oxygen therapy (and have Pao2 results of less than 55 mm Hg). Oxygen therapy will extend the lives of hypoxemic COPD patients, decrease hematocrit, improve neuropsychological performance, and improve pulmonary hemodynamics. Oxygen will decrease dyspnea, minute ventilation, and work of breathing; 24-hour oxygen therapy in patients with less severe hypoxemia does not prolong survival. Nocturnal oxygen therapy for COPD patients with sleep-related oxygen desaturation but mild-to-moderate daytime hypoxemia does not improve survival. Nocturnal oxygen therapy does not delay the need for continuous long-term oxygen. There is a small improvement in neuropsychological function and quality of life associated with nocturnal oxygen therapy.
Whether nocturnal oxygen therapy improves sleep quality is unknown.21 There is some suggestion that REM sleep increases when oxygen is administered.
Positive-Pressure Ventilation
Hypoventilation plays a significant role in sleep-related oxygen desaturation in COPD, even without OSA. Noninvasive methods of ventilation will decrease work of breathing and treat nocturnal hypoxemia in stable, severe COPD patients. Ventilation can be provided via nasal or facial mask using a conventional ventilator or a bilevel positive airway pressure machine.
Perrin et al22 reported that a combination of nocturnal oxygen and nocturnal intermittent positive-pressure ventilation (NIPPV), used for 6 months, significantly improved ABG levels and the quality of life in stable, hypercapnic COPD patients. No significant changes were reported in overall mortality, pulmonary function, hemodynamics, hospitalization, or need for intubation.
In a study by Leger et al,23 50 COPD patients received supplemental oxygen plus NIPPV and were followed for 5 years. The patients showed a significant reduction in Paco2 during spontaneous ventilation in the first and second years of treatment, but no related improvement in Pao2. The factors that contribute to improvement in ABG results may include reduced work of breathing, increased chemosensitivity to carbon dioxide, and re-expansion of microatelectatic lung segments through positive-pressure ventilation.
In many investigations,21,24 inspiratory pressure was titrated to achieve comfort, decreased dyspnea, a respiratory rate of 12 to 14 breaths per minute, and decreased use of accessory respiratory muscles. The inspiratory pressures used were 8 to 12 cm H2O, with compensation for intrinsic positive end-expiratory pressure. The expiratory pressures used were 4 to 5 cm H2O.
In patients with severe COPD and OSA, treatment with nocturnal continuous positive airway pressure (CPAP) produced improvement in lung function during wakefulness. Mansfield and Naughton25 studied 14 patients with COPD and OSA (defined as an apnea/hypopnea index of more than five episodes per hour). The main findings of the study were a significant reduction in Paco2, a rise in Pao2, and an improvement in FEV1 associated with a fall in the hospitalization rate.
Hypercapnic COPD patients treated with NIPPV had improved sleep quality, increased total sleep time, and improved sleep efficiency without a change in sleep architecture or arousal.26 Reduced work of breathing and an associated decrease in central respiratory drive, along with improved nocturnal gas exchange during NIPPV, accounted for the improvements in sleep efficiency and total sleep time.
One study27 evaluated the effect of tracheostomy in patients with overlap syndrome. They showed persistent nocturnal oxygen desaturation, but improvement in right-sided hemodynamics. Most of these patients also received long-term nocturnal oxygen therapy.
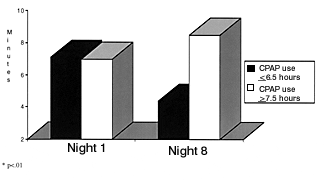
Serial polysomnography in patients with overlap syndrome showed that patients required higher CPAP levels to eliminate apnea when oxygen was administered, compared with CPAP alone.28 This indicates a worsening of sleep apnea when patients with COPD and OSA are given oxygen alone. It seems possible that nasal bilevel positive airway pressure could provide an additional benefit in terms of maintaining upper-airway patency and augmenting nocturnal ventilation.
When the coexistence of COPD and OSA is likely, the patient benefits from polysomnography. There is no evidence to support the need for polysomnography for every patient with COPD and nocturnal oxygen desaturation. The American Thoracic Society29 recommends polysomnography for COPD patients with a diurnal Pao2 of more than 55 mm Hg and evidence of cor pulmonale, pulmonary hypertension, or polycythemia, as well as for any patient, with or without COPD, who has symptoms of OSA.
Mehrgan Sokhandan, MD, is in private practice and is also affiliated with Southeast Regional Sleep Disorders Center, Greenville, SC. Freddie E. Wilson, MD, is medical director at the center.
REFERENCES
1. Mannino DM, Gagnon RC, Peggy TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683-1689.
2. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269-280.
3. Anto J, Vermeire P, Vestbo J, Sunyer J. Epidemiology of chronic obstructive pulmonary disease. Eur Respir J. 2001;17:982-984.
4. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77-S121.
5. Ferguson GT, Enright PL, Buist AS, Higgins MW. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146-1161.
6. Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease: the Lung Health Study. Am J Respir Crit Care Med. 2000;161:381-390.
7. Fletcher EC, Miller J, Devine GW, et al. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tension above 60 mm Hg. Chest. 1987;92:604-608.
8. Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease, a clinical trial. Ann Intern Med. 1980;93:391-398.
9. Chaoust A, Weltzenblum E, Krieger J, et al. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med. 1995;15:62-85.
10. Fischer D, Raschke F. Incidence of obstructive sleep apnea syndrome in combination with COPD. Pneumologie. 1992;47:731-734.
11. Guilleminault C, Cummiskey J, Motta J. Chronic obstructive airflow disease and sleep studies. Am Rev Respir Dis. 1980;122:397-405.
12. Radwan L, Maszczyk Z, Koziorowski A. Control of breathing in obstructive sleep apnea and in patients with the overlap syndrome. Eur Respir J. 1995;8:542-545.
13. Man GGW, Chapman KR, Ali SM. Sleep quality and nocturnal respiratory function in once-daily theophylline (uniphyl) and inhaled salbutamol in patients with COPD. Chest. 1996;110:648-653.
14. Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe COPD. Am Rev Respir Dis. 1993;148:1030-1036.
15. Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;I:681-686.
16. Howard P, de Haller R. Domiciliary oxygen by liquid or concentrator. Eur Respir J. 1991;4:1284-1287.
17. Timms RM, Khaja FU, Williams GW. Hemodynamic response to oxygen therapy in chronic obstructive pulmonary disease. Ann Intern Med. 1985;102:29-36.
18. Flenley DC, Douglas NJ, Lamb D. Nocturnal hypoxemia and long term domiciliary O2 in blue and bloated bronchitis. Chest. 1980;77:305-307.
19. Gorecka D, Gorzelah K, Sliwinski P, Tobiasz M, Zielinski J. Effect of long term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxemia. Thorax. 1997;52:674-679.
20. Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime Pao2 above 60 mm Hg. Am Rev Respir Dis. 1992;145:1070-1076.
21. Calvarey PMA, Brezinova V, Douglas NJ, et al. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126:206-210.
22. Perrin C, Far EL, Vandenbos F, et al. Domiciliary nasal intermittent positive pressure ventilation in severe COPD: effect on lung function and quality of life. Eur Respir J. 1997;10:2835-2839.
23. Leger P, Bedicam JM, Cornette A, et al. Nasal intermittent positive pressure ventilation; long term follow-up in patients with severe chronic respiratory insufficiency. Chest. 1994;105:100-105.
24. Casanova C, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest. 2000;118:1582-1590.
25. Mansfield D, Naughton M. Effect of continuous positive airway pressure on lung function in patients with COPD and sleep disordered breathing. Respirology. 1999;4:365-370.
26. Krochman S, Quaranta A. Effect of NIPPV on gas exchange in COPD patients. Chest. 1997;112:623-628.
27. Fletcher KC, Brown DL. Nocturnal oxyhemoglobin desaturation following tracheostomy for obstructive sleep apnea. Am J Med. 1985;79:35-42.
28. Sampol G, Sagales MT, Roca A, et al. Nasal continuous positive airway pressure with supplemental oxygen in coexistent sleep apnea-hypopnea syndrome and severe chronic obstructive pulmonary disease. Eur Respir J. 1996;9:111-116.
29. American Thoracic Society. Indications and standards for cardiopulmonary sleep studies. Am Rev Respir Dis. 1989;139:559-568.