Jazz Pharmaceuticals plc presented positive efficacy results from its global multicenter studies (TONES 3 and TONES 4) of JZP-110 in adult patients with excessive sleepiness associated with obstructive sleep apnea (OSA). The data were presented in a poster session at SLEEP 2017 meeting in Boston.
“Obstructive sleep apnea (OSA) is a serious chronic sleep disorder in which breathing repeatedly stops and starts during sleep,” says Karen Smith, MD, PhD, executive vice president of research and development and chief medical officer of Jazz Pharmaceuticals, in a release. “These studies suggest that JZP-110 could provide an important treatment option for patients with excessive sleepiness with OSA who may not respond to, or may experience inadequate response to, their current wake-promoting therapies. It also underscores our commitment to making significant research and development investments to find innovative treatments for unmet medical needs in sleep medicine.”
“People who experience excessive sleepiness associated with OSA may have an inadequate response to attempts to treat the OSA, underscoring the need for new treatment options for residual sleepiness that may affect their quality of life,” says Kingman Strohl, MD, professor of medicine, University Hospitals Case Medical Center and Case School of Medicine, Cleveland, Ohio. “The results with JZP-110 show significant increase in a patients’ ability to stay awake and decreased patients’ subjective levels of sleepiness compared to placebo, in a rather customary practice setting.”
The Treatment of OSA and Narcolepsy Excessive Sleepiness (TONES) Phase 3 program is comprised of four studies, one study evaluating excessive sleepiness in adult patients with narcolepsy (TONES 2), two studies evaluating excessive sleepiness in adult patients with OSA (TONES 3 and TONES 4), and an open-label, long-term safety and maintenance of efficacy study (TONES 5) in the treatment of excessive sleepiness in patients with narcolepsy or OSA.
About the Global Phase 3 TONES 3 Study
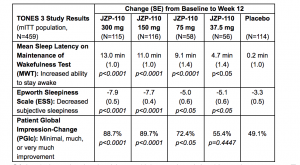
TONES 3 was a 12-week, 5-arm, parallel-group, double-blind, placebo-controlled, randomized Phase 3 study that evaluated the safety and efficacy of JZP-110 at 300 mg, 150 mg, 75 mg, and 37.5 mg compared to placebo, in adults with excessive sleepiness in OSA. Patients (N=476) were randomized 1:1:2:2:2 to JZP-110 37.5 mg (n=59), 75 mg (n=61), 150 mg (n=118), 300 mg (n=119), or placebo (n=119). The co-primary endpoints were the change in mean sleep latency on the Maintenance of Wakefulness Test (MWT) and in the Epworth Sleepiness Scale (ESS) score, from baseline to week 12. The key secondary endpoint was the percentage of patients who reported improvement on the Patient Global Impression of Change (PGIc) scale, a patient-reported measure of overall condition from baseline to week 12.
In adult patients with OSA, JZP-110 at all doses demonstrated statistically significant improvements on the co-primary endpoints of mean sleep latency on the MWT and the ESS from baseline to week 12 compared to placebo. The endpoints of MWT and ESS measure patients’ ability to stay awake and patients’ subjective levels of sleepiness, respectively. JZP-110 increased the mean MWT sleep latency by more than 10 minutes at all time points at the 150 and 300 mg doses. JZP-110 decreased mean ESS scores by more than 7 points at the 150 and 300 mg doses at week 12.
On the key secondary endpoint of the PGIc scale, patients reported significant overall improvement compared to placebo at week 12 on all doses of JZP-110 except for 37.5 mg. Approximately 90% of patients reported overall improvement on the 150 and 300 mg doses at week 12.
Results from the primary analysis of the co-primary endpoints of MWT and ESS, and analysis of the key secondary endpoint of PGIc were:
Of the 476 randomized subjects, 404 completed the 12-week treatment. Twenty-nine patients (6.1%) in the safety population: four patients (3.4%) on placebo and 25 (7.0%) on JZP-110, discontinued due to treatment emergent adverse events. The most commonly reported treatment emergent adverse events (occurring in >5% of patients across all doses of JZP-110) in the TONES 3 study were headache, nausea, decreased appetite, anxiety, and nasopharyngitis. There were seven serious treatment emergent adverse events reported in five patients: goiter (n=1), and a motor vehicle accident, back pain, and sciatica (n=1) in two patients on placebo; bile duct obstruction (n=1) and streptococcal endocarditis (n=1) in two patients on JZP-110 37.5 mg; and hyperglycemia (n=1) in one patient on JZP-110 150 mg. One subject had a non-treatment emergent SAE of coronary artery disease that started prior to receiving JZP-110 300 mg and that was not reported until after dosing. Treatment emergent adverse events and discontinuations due to an adverse events were most common at the JZP-110 300 mg dose.
About the Global Phase 3 TONES 4 Study
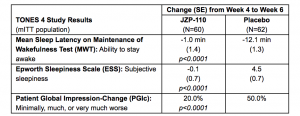
TONES 4 was a six-week Phase 3 study comprising a two-week flexible-dose titration phase followed by two-weeks of stable dose treatment. Patients who reported “much” or “very much” improvement and who had numerical improvements on the MWT and ESS at the end of the stable dose phase (week 4) then entered a two-week, placebo-controlled, double-blind randomized withdrawal phase. In this study, 174 patients were titrated to a maximum tolerated dose over a two-week period, and 157 patients continued on that dose for two weeks in the stable dose phase. The primary analysis (n=122) in the modified intent to treat (mITT) population) evaluated the difference between JZP-110 treatment versus placebo on the co-primary endpoints of MWT and ESS, measured from the end of the stable dose phase at week 4 to the end of the randomized withdrawal phase at week 6.
Patients who completed the 4-week treatment and remained on JZP-110 did not demonstrate loss of efficacy relative to those who were randomized to placebo.
Results from the primary analysis of the co-primary endpoints of MWT and ESS, and analysis of the key secondary endpoint of PGIc were:
During the double-blind withdrawal phase (weeks 4 to 6), patients who continued on JZP-110 remained improved on the MWT and ESS. Patients who switched to placebo showed worsening of sleepiness, with mean MWT sleep latency decreased by 12.1 minutes from week 4 to week 6 (compared with a decrease of 1.0 minute for those who remained on JZP-110, p<0.0001) and mean ESS scores increased by 4.5 minutes (compared to a mean decrease of 0.1 minutes for those who stayed on JZP-110, p<0.0001).
A significantly higher percentage of patients who were switched to placebo experienced a worsening of their overall condition on the PGIc as compared with patients who stayed on JZP-110 (p<0.0001).
There were no serious treatment emergent adverse events. Six patients (3.4%) withdrew from the study due to treatment emergent adverse events. Most treatment emergent adverse events occurred during the titration phase of the study. The most common treatment emergent adverse events (occurring in ?5% patients) during the titration phase were headache, dry mouth, nausea, dizziness, and insomnia.
JZP-110 is a selective dopamine and norepinephrine reuptake inhibitor (DNRI) in development for treatment of excessive sleepiness in adult patients with narcolepsy, OSA, and Parkinson’s disease. In 2014, Jazz Pharmaceuticals acquired a license to develop and commercialize JZP-110 from SK Biopharmaceuticals, which discovered the compound. Jazz Pharmaceuticals has worldwide development, manufacturing, and commercialization rights to JZP-110, excluding certain jurisdictions in Asia. SK Biopharmaceuticals maintains rights in Korea, Japan, China, Taiwan, Singapore, Indonesia, India, Philippines, Thailand, Malaysia, Vietnam, and Hong Kong. JZP-110 has orphan drug designation in the United States for narcolepsy.
Across the entire JZP-110 development program, over 2,000 subjects have enrolled in 20 studies. The JZP-110 Phase 3 clinical program includes one study evaluating excessive sleepiness in adult patients with narcolepsy (TONES 2), two studies evaluating excessive sleepiness in adult patients with OSA (TONES 3 and TONES 4), and an open-label, long-term safety and maintenance of efficacy study (TONES 5) in the treatment of excessive sleepiness in patients with narcolepsy or OSA. Enrollment is complete in all studies that are expected to support Jazz Pharmaceuticals’ planned JZP-110 New Drug Application (NDA) submission to the U.S. Food and Drug Administration (FDA) in late 2017.